 Briefly, Cranial Electrotherapy Stimulation (CES) utilizes extremely small levels of electrical stimulation across the head. It has been found to be efficacious for a number of difficulties including anxiety, depression, insomnia, and chronic pain. CES units are approved in the United States by the Federal Drug Administration (FDA) for the treatment of anxiety, depression, and insomnia. Click here and here to learn more. Click here for a list of references.
Briefly, Cranial Electrotherapy Stimulation (CES) utilizes extremely small levels of electrical stimulation across the head. It has been found to be efficacious for a number of difficulties including anxiety, depression, insomnia, and chronic pain. CES units are approved in the United States by the Federal Drug Administration (FDA) for the treatment of anxiety, depression, and insomnia. Click here and here to learn more. Click here for a list of references.
Evidence from published research suggests CES use activates particular groups of nerve cells that produce the neurotransmitters serotonin and acetylcholine which can affect the chemical activity of nerve cells that are both nearby and distant in the nervous system. By changing the electrical and chemical activity of certain nerve cells, CES devices like Alpha-Stim® (seen on the left) appear to amplify activity in some neurological systems and deactivate activity in others. This neurological ‘fine tuning’ occurs either as a result of, or together with the production of a certain type of electrical activity pattern in the brain known as an alpha state. CES has been found increase alpha and decrease delta, theta, and beta waves on qEEG brain maps. This typically results in feeling calm, relaxed and focused. This appears to decrease stress effects, reduce agitation, stabilize mood, and regulate both sensations and perceptions of particular types of pain.
Adapted from Alpha-Stim.com
According to the Johns Hopkins School of Medicine, “tDCS is a non-invasive, painless brain stimulation treatment that uses direct electrical currents to stimulate specific parts of the brain. A constant, low intensity current is passed through two electrodes placed over the head which modulates neuronal activity. There are two types of stimulation with tDCS: anodal and cathodal stimulation. Anodal stimulation acts to excite neuronal activity while cathodal stimulation inhibits or reduces neuronal activity.
Although tDCS is still an experimental form of brain stimulation, it potentially has several advantages over other brain stimulation techniques. It is cheap, non-invasive, painless and safe. It is also easy to administer and the equipment is easily portable. The most common side effect of tDCS is a slight itching or tingling on the scalp.
Several studies suggest it may be a valuable tool for the treatment of neuropsychiatric conditions such as depression, anxiety, Parkinson’s disease, and chronic pain. Research has also demonstrated cognitive improvement in some patients undergoing tDCS. Currently, tDCS is not an FDA-approved treatment.”
 tDCS involves the application of a constant, low current to the brain area of interest via electrodes on the head. It is believed to increase or decrease neural excitability (depending on the electrode configuration). tDCS is currently an experimental treatment for various conditions such as depression, anxiety, and brain injuries including strokes. Research to date suggests there are no serious side-effects or reported adverse events (click here for a recent review of adverse events). There is evidence that tDCS can improve psychological problems such as depression and possibly anxiety (click here, here and here). In addition, there is evidence that tDCS can improve cognitive performance in otherwise healthy subjects (click here and here) and possibly enhance cognitive functions in those with brain damage (click here). For a 2014 review of the research click here. For a 2016 review of the research, click here.
tDCS involves the application of a constant, low current to the brain area of interest via electrodes on the head. It is believed to increase or decrease neural excitability (depending on the electrode configuration). tDCS is currently an experimental treatment for various conditions such as depression, anxiety, and brain injuries including strokes. Research to date suggests there are no serious side-effects or reported adverse events (click here for a recent review of adverse events). There is evidence that tDCS can improve psychological problems such as depression and possibly anxiety (click here, here and here). In addition, there is evidence that tDCS can improve cognitive performance in otherwise healthy subjects (click here and here) and possibly enhance cognitive functions in those with brain damage (click here). For a 2014 review of the research click here. For a 2016 review of the research, click here.
Transcranial Photobiomodulation (aka Neurophotomodulation) or low-level light therapy (LLLT) involves the use of low-level near infrared light to stimulate neuronal mitochondria and cellular events. These lights are applied to the patient’s head for therapeutic purposes such as recovery from neurological disorder or damage. Light in the red and near-infrared regions of the electromagnetic spectrum are used because of their ability to penetrate the scalp, skull and brain. Studies have been performed to assess the safety and effectiveness of transcranial light therapy in conditions such as stroke, traumatic brain injury (TBI), and neurodegenerative conditions.
For a review of the research, click here (general review), here (Alzheimer’s and Parkinson’s disease), here (TBI), here (mild TBI/concussions) here (improved cognitive and mood in healthy subjects), and here (references).
The first study to report significant cognitive improvement in dementia participants following brain photobiomodulation treatments was presented at the Alzheimer’s Association International Conference in Toronto in July 2016. To see the abstract, click here. To see the poster, click here. To see a related news clip click here.
Due to the fact that only recently have researchers started to use transcranial photobiomodulation in the treatment of brain disorders and, although transcranial photobiomodulation is considered safe, at this point in time it is considered experimental and thus no medical claims can be made.





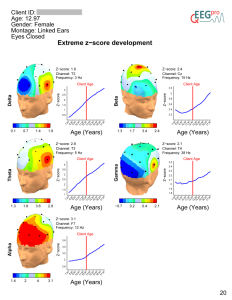
 Quantitative Electroencephalography (qEEG) is a procedure that processes the recorded EEG activity from a multi-electrode recording using a computer. The digital data is statistically analyzed, comparing values with “normative” database reference values. The processed EEG is commonly converted into color maps of brain functioning called “Brain Maps”.
Quantitative Electroencephalography (qEEG) is a procedure that processes the recorded EEG activity from a multi-electrode recording using a computer. The digital data is statistically analyzed, comparing values with “normative” database reference values. The processed EEG is commonly converted into color maps of brain functioning called “Brain Maps”.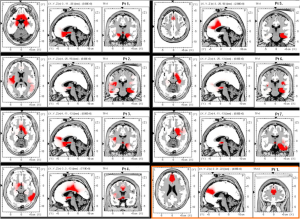
 Briefly, Cranial Electrotherapy Stimulation (CES) utilizes extremely small levels of electrical stimulation across the head. It has been found to be efficacious for a number of difficulties including anxiety, depression, insomnia, and chronic pain. CES units are approved in the United States by the Federal Drug Administration (FDA) for the treatment of anxiety, depression, and insomnia. Click
Briefly, Cranial Electrotherapy Stimulation (CES) utilizes extremely small levels of electrical stimulation across the head. It has been found to be efficacious for a number of difficulties including anxiety, depression, insomnia, and chronic pain. CES units are approved in the United States by the Federal Drug Administration (FDA) for the treatment of anxiety, depression, and insomnia. Click  tDCS involves the application of a constant, low current to the brain area of interest via electrodes on the head. It is believed to increase or decrease neural excitability (depending on the electrode configuration). tDCS is currently an experimental treatment for various conditions such as depression, anxiety, and brain injuries including strokes. Research to date suggests there are no serious side-effects or reported adverse events (click
tDCS involves the application of a constant, low current to the brain area of interest via electrodes on the head. It is believed to increase or decrease neural excitability (depending on the electrode configuration). tDCS is currently an experimental treatment for various conditions such as depression, anxiety, and brain injuries including strokes. Research to date suggests there are no serious side-effects or reported adverse events (click 
